 |
 |

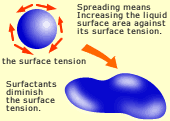 The boundary where two of the solid, liquid or gas phases contact with each other is called the "interface". A squeezing force (called the interface tension, or when the phase is gas, it is called the surface tension), which attempts to maintain the contact area of two phases as small as possible, works on the interface. The boundary where two of the solid, liquid or gas phases contact with each other is called the "interface". A squeezing force (called the interface tension, or when the phase is gas, it is called the surface tension), which attempts to maintain the contact area of two phases as small as possible, works on the interface.
Such a phenomenon that a drop of water on a glass plate does not extend its area but stays round like a convex lens is due to the surface tension. (A volume has its smallest surface area when it is in a sphere shape.)
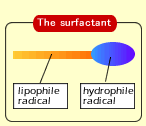
Such a matter that collects on the interface and works to reduce the interface tension remarkably is called the surface-active agent or the surfactant. A surfactant has in its singular molecule "the portion that gets along well with water (hydrophile radical)" and "the portion that gets along well with grease (lipophile radical)", and has the property of both of the radicals. |
|
 |
 |
 |
 |

Due to the chemical structure having both the hydrophile radical and the lipophile radical in a single molecule, a surfactant has unique characteristics.
Described hereafter are the basic ones of such characteristics.
(1) Reduction of surface tension (interface tension)
While a drop of plain water on glass makes a round drop, a liquid containing a small amount of surfactant like soap extends on the surface. As you shake water in a bottle, bubbles occur but disappear quickly. If a small amount of surfactant is added, large bubbles occur and will not disappear for a while. A surfactant also allows to emulsify oil or to disperse fine particles of a solid material. Those phenomena occur because the surfactant reduces the surface tension (in case of water and oil, it is the interface tension) of the water, causing the surface area of the water to increase.
(2) Absorption and forming of micelle
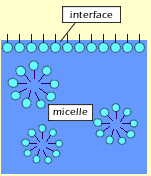
The surfactant dissolved in water has a tendency to collect on the surface instead of staying inside. As such, the tendency to collect on the surface instead of staying inside is called the absorption. As you further increase the concentration of surfactant in the water, the surface is saturated with the surfactant molecules, thus the surfactant creates a herd of hydrophile radicals (micelle) with the hydrophile radicals facing toward the water. (The concentration that causes the aforementioned phenomenon is called the critical micelle concentration.) As micelle is formed, even non-water-soluble oil can be made dissolvable in water (solutizing).
The surfactant has the above-mentioned basic characteristics that bring about various effects by one or a combination of such characteristics.
The surfactant also is absorbed in the surface of a solid material. That nature of a surfactant is applied mainly for purposes such as lubrication, anti-static, etc. In case of cosmetics and the likes, a surfactant is sometimes used to keep moisture on the surface of skin to attain a tender touch. In such an application, the surfactant is called the emollient agent.
Nihon Emulsion newly developed the amino acid base surfactants AMITER and PYROTER, which are highly reputed as emollient agents to mix in cosmetics.
|
|
 |
 |
|


